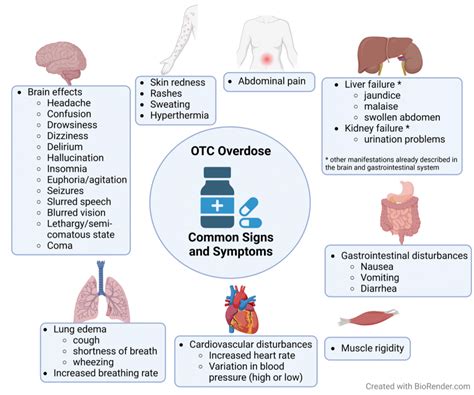
A common over-the-counter (OTC) nasal decongestant, pseudoephedrine, found in medications like Sudafed and some generic brands, has been linked to a small increased risk of serious neurological side effects, including stroke and reversible cerebral vasoconstriction syndrome (RCVS), prompting safety reviews by regulatory agencies.
The Food and Drug Administration (FDA) is evaluating new data concerning pseudoephedrine and its potential association with ischemic stroke and hemorrhagic stroke. This review follows similar concerns raised about phenylephrine, another common decongestant, which the FDA recently determined to be ineffective when taken orally. The growing scrutiny of common decongestants has raised concerns among consumers who rely on these medications to relieve nasal congestion caused by allergies, colds, and sinus infections.
The FDA announced in December 2023 that it would investigate the risk of RCVS and posterior reversible encephalopathy syndrome (PRES) associated with pseudoephedrine-containing products. These rare but serious conditions affect blood vessels in the brain and can lead to stroke-like symptoms, headaches, and visual disturbances. The agency’s investigation was prompted by reports submitted to the FDA Adverse Event Reporting System (FAERS) and published cases in medical literature.
While the absolute risk of stroke or RCVS remains low, the FDA emphasizes the importance of awareness and caution among users and healthcare providers. The agency advises individuals to seek immediate medical attention if they experience sudden and severe headaches, visual disturbances, seizures, or neurological changes after taking pseudoephedrine.
The Consumer Healthcare Products Association (CHPA), which represents manufacturers of OTC medications, said it is working with the FDA to provide information and ensure the safe use of pseudoephedrine products. The CHPA emphasized that pseudoephedrine has been used safely and effectively for decades when used as directed.
Concerns Over Neurological Risks
Pseudoephedrine is a sympathomimetic amine, meaning it mimics the effects of adrenaline and noradrenaline in the body. It works by constricting blood vessels in the nasal passages, which reduces swelling and congestion. However, this vasoconstrictive effect can also affect blood vessels in other parts of the body, including the brain.
RCVS is a condition characterized by temporary narrowing of blood vessels in the brain, which can restrict blood flow and cause stroke-like symptoms. PRES is a related condition that involves swelling in the brain, also potentially leading to neurological complications.
The FDA’s ongoing investigation is focused on determining the strength of the association between pseudoephedrine and these conditions. The review will consider the frequency of reported cases, the severity of the outcomes, and the presence of other risk factors that may contribute to the development of RCVS and PRES.
“We are evaluating all available data and will communicate any new information to the public as soon as possible,” an FDA spokesperson stated.
Phenylephrine Ineffectiveness
The current concerns about pseudoephedrine come on the heels of the FDA’s determination that oral phenylephrine, another widely used decongestant, is ineffective. In September 2023, the FDA’s Nonprescription Drugs Advisory Committee unanimously concluded that available data do not support the efficacy of oral phenylephrine at the doses currently available in OTC products.
Phenylephrine is often used as a substitute for pseudoephedrine in OTC decongestants because it is less regulated. Pseudoephedrine is subject to stricter regulations due to its potential use in the illegal production of methamphetamine. However, the FDA’s determination of phenylephrine’s ineffectiveness has left consumers with fewer options for treating nasal congestion.
The ineffectiveness of oral phenylephrine has led to increased scrutiny of other decongestants and calls for more rigorous testing and regulation of OTC medications. Consumer advocacy groups have urged the FDA to take swift action to remove ineffective products from the market and ensure that consumers have access to safe and effective treatments.
Alternatives to Pseudoephedrine and Phenylephrine
Given the potential risks associated with pseudoephedrine and the ineffectiveness of oral phenylephrine, consumers may want to consider alternative treatments for nasal congestion.
Several non-medication options can help relieve congestion, including:
- Nasal saline sprays: These sprays help to moisturize the nasal passages and loosen mucus, making it easier to breathe.
- Nasal irrigation: Using a neti pot or other nasal irrigation device can help to flush out the nasal passages and remove irritants.
- Steam inhalation: Inhaling steam from a hot shower or a bowl of hot water can help to open up the nasal passages.
- Humidifiers: Using a humidifier can help to add moisture to the air, which can prevent the nasal passages from drying out.
In addition to these non-medication options, some other medications can help relieve nasal congestion, including:
- Topical nasal decongestants: These medications, such as oxymetazoline (Afrin), work by constricting blood vessels in the nasal passages. However, they should only be used for a short period of time (usually no more than three days) to avoid rebound congestion.
- Antihistamines: These medications can help relieve congestion caused by allergies.
- Corticosteroid nasal sprays: These sprays can help reduce inflammation in the nasal passages and relieve congestion.
It is important to talk to a healthcare provider before using any new medication, especially if you have any underlying health conditions or are taking other medications.
Expert Opinions and Recommendations
Medical experts emphasize that the risk of stroke or RCVS associated with pseudoephedrine is small, but they also stress the importance of being aware of the potential risks and seeking medical attention if symptoms develop.
“The key message is that these are rare events,” said Dr. [Fictional Name], a neurologist at [Fictional Hospital]. “But it’s important for people to be aware of the potential risks and to seek medical attention if they experience any concerning symptoms.”
Dr. [Fictional Name] recommends that people with a history of high blood pressure, heart disease, or stroke should use pseudoephedrine with caution and consult with their healthcare provider before taking it.
Other experts suggest that consumers consider alternative treatments for nasal congestion, especially if they have risk factors for cardiovascular disease or stroke.
“There are many effective ways to relieve nasal congestion without using pseudoephedrine,” said Dr. [Fictional Name], a family medicine physician at [Fictional Clinic]. “I encourage my patients to try non-medication options first, and if they need medication, to consider alternatives like saline sprays or antihistamines.”
Regulatory Landscape and Future Actions
The FDA’s ongoing investigation into pseudoephedrine highlights the importance of post-market surveillance of OTC medications. Post-market surveillance allows regulatory agencies to monitor the safety and effectiveness of medications after they have been approved and are available to the public.
The FDA’s review of pseudoephedrine may lead to new labeling requirements, restrictions on access to the medication, or even a recall of certain products. The agency will consider all available data and expert opinions before making a final decision.
The CHPA has stated that it is committed to working with the FDA to ensure the safe use of pseudoephedrine products. The association said that it will continue to provide information to consumers and healthcare providers about the risks and benefits of pseudoephedrine.
Impact on Consumers
The concerns about pseudoephedrine and the ineffectiveness of oral phenylephrine have left many consumers feeling confused and frustrated. Consumers rely on OTC medications to relieve common symptoms like nasal congestion, and they expect these medications to be both safe and effective.
The FDA’s actions highlight the importance of reading labels carefully and talking to a healthcare provider before using any new medication. Consumers should also be aware of the potential risks and benefits of different treatments and make informed decisions about their healthcare.
The uncertainty surrounding decongestants may also lead consumers to seek out alternative treatments, such as herbal remedies or homeopathic products. However, it is important to note that these products are not regulated by the FDA and may not be safe or effective.
The Broader Context of OTC Medication Safety
The situation with pseudoephedrine and phenylephrine underscores broader concerns about the safety and regulation of OTC medications. While OTC drugs are generally considered safe when used as directed, they are not without risks. The sheer volume of OTC medications consumed, coupled with the potential for misuse or interactions with other drugs, means that adverse events can and do occur.
The FDA plays a crucial role in ensuring the safety and effectiveness of OTC medications, but its resources are limited, and it relies heavily on post-market surveillance to identify potential problems. The reporting of adverse events by consumers and healthcare professionals is essential for effective post-market surveillance.
The OTC drug market is constantly evolving, with new products and formulations being introduced regularly. This rapid pace of innovation can make it challenging for regulatory agencies to keep up and ensure that all products meet safety and efficacy standards.
Conclusion
The link between pseudoephedrine and potential neurological harms, coupled with the demonstrated ineffectiveness of oral phenylephrine, presents a complex challenge for consumers and healthcare providers. While the absolute risk associated with pseudoephedrine appears to be low, the potential for serious adverse events warrants caution and awareness. Consumers should carefully consider the risks and benefits of pseudoephedrine before using it and should be aware of alternative treatments for nasal congestion. The FDA’s ongoing investigation and the broader concerns about OTC medication safety highlight the importance of informed decision-making and responsible self-care. As the regulatory landscape evolves, it is crucial for consumers to stay informed and to consult with healthcare professionals to ensure they are using safe and effective treatments. The situation also underscores the need for continued research and vigilance in monitoring the safety of widely used OTC medications. The ultimate goal is to provide consumers with access to treatments that are both effective and safe, allowing them to manage common ailments without undue risk.
Frequently Asked Questions (FAQ)
1. What is pseudoephedrine, and why is it used?
Pseudoephedrine is a decongestant medication that works by narrowing blood vessels in the nasal passages, reducing swelling and congestion. It is commonly used to relieve nasal congestion caused by allergies, colds, and sinus infections. It’s a sympathomimetic amine, meaning it mimics the effects of adrenaline and noradrenaline.
2. What are the potential neurological risks associated with pseudoephedrine?
The FDA is investigating a possible link between pseudoephedrine and rare but serious neurological side effects, including ischemic stroke, hemorrhagic stroke, reversible cerebral vasoconstriction syndrome (RCVS), and posterior reversible encephalopathy syndrome (PRES). These conditions affect blood vessels in the brain and can lead to stroke-like symptoms, headaches, and visual disturbances.
3. How significant is the risk of experiencing these neurological side effects from pseudoephedrine?
While the FDA is investigating the link, it is important to note that the absolute risk of stroke or RCVS associated with pseudoephedrine is considered low. However, individuals should be aware of the potential risks and seek medical attention if they experience any concerning symptoms after taking pseudoephedrine.
4. What are the alternatives to pseudoephedrine for treating nasal congestion?
There are several alternatives to pseudoephedrine, including:
- Nasal saline sprays: Moisturize the nasal passages and loosen mucus.
- Nasal irrigation (Neti pot): Flushes out the nasal passages.
- Steam inhalation: Opens up nasal passages.
- Humidifiers: Adds moisture to the air.
- Topical nasal decongestants (oxymetazoline): Should be used for a short period only (no more than three days).
- Antihistamines: For congestion caused by allergies.
- Corticosteroid nasal sprays: Reduce inflammation.
Also, oral phenylephrine was a common alternative. However, FDA recently determined it ineffective when taken orally.
5. What should I do if I experience symptoms like a sudden headache or visual disturbances after taking pseudoephedrine?
If you experience sudden and severe headaches, visual disturbances, seizures, or neurological changes after taking pseudoephedrine, seek immediate medical attention. These symptoms could be indicative of a serious neurological condition like RCVS or PRES.
6. What is the FDA doing about the potential risks associated with pseudoephedrine?
The FDA is currently evaluating new data concerning pseudoephedrine and its potential association with ischemic stroke and hemorrhagic stroke. This review follows similar concerns raised about phenylephrine, another common decongestant, which the FDA recently determined to be ineffective when taken orally. The FDA is reviewing reports submitted to the FDA Adverse Event Reporting System (FAERS) and published cases in medical literature. They will communicate any new information to the public as soon as possible.
7. Should I stop taking pseudoephedrine immediately?
You should consult with your healthcare provider before stopping any medication, including pseudoephedrine. They can assess your individual risk factors and provide guidance on the best course of action. If you have a history of high blood pressure, heart disease, or stroke, it is especially important to discuss the risks and benefits of pseudoephedrine with your doctor.
8. What is the Consumer Healthcare Products Association (CHPA) saying about the issue?
The CHPA, which represents manufacturers of OTC medications, is working with the FDA to provide information and ensure the safe use of pseudoephedrine products. They emphasize that pseudoephedrine has been used safely and effectively for decades when used as directed.
9. What is the difference between pseudoephedrine and phenylephrine?
Both pseudoephedrine and phenylephrine are decongestants used to relieve nasal congestion. However, pseudoephedrine is more effective than phenylephrine. Phenylephrine is often used as a substitute for pseudoephedrine in OTC decongestants because it is less regulated due to pseudoephedrine’s potential use in illegal methamphetamine production. However, the FDA has determined that oral phenylephrine is ineffective at the doses currently available in OTC products.
10. What are RCVS and PRES?
RCVS (Reversible Cerebral Vasoconstriction Syndrome) is a condition characterized by temporary narrowing of blood vessels in the brain, which can restrict blood flow and cause stroke-like symptoms. PRES (Posterior Reversible Encephalopathy Syndrome) is a related condition that involves swelling in the brain, also potentially leading to neurological complications. Both are rare but serious conditions.
11. Does this news affect all products that contain pseudoephedrine?
The FDA’s investigation concerns all products containing pseudoephedrine, including brand-name medications like Sudafed and generic versions. It is the ingredient itself that is under review, not specific brands.
12. Are children at greater risk than adults from pseudoephedrine?
The FDA has not specifically stated that children are at greater risk, but the general recommendation is that parents should always consult a pediatrician before giving any OTC medication to a child. This is because children may be more susceptible to side effects from medications. Furthermore, dosage adjustments are crucial for children.
13. Where can I report an adverse event related to pseudoephedrine?
You can report an adverse event related to pseudoephedrine to the FDA through the FDA Adverse Event Reporting System (FAERS). This information helps the FDA monitor the safety of medications and identify potential problems.
14. How does pseudoephedrine affect blood pressure?
Pseudoephedrine can increase blood pressure due to its vasoconstrictive properties. It is a sympathomimetic amine, meaning it mimics the effects of adrenaline and noradrenaline, which can lead to increased heart rate and blood pressure. Therefore, individuals with high blood pressure should use pseudoephedrine with caution and consult their doctor.
15. Can pseudoephedrine interact with other medications?
Yes, pseudoephedrine can interact with other medications, including certain antidepressants (MAOIs), blood pressure medications, and other stimulants. These interactions can increase the risk of side effects or reduce the effectiveness of the medications. It is important to inform your doctor about all the medications you are taking before using pseudoephedrine.
16. What does “post-market surveillance” mean in the context of OTC drugs?
Post-market surveillance refers to the monitoring of the safety and effectiveness of medications after they have been approved and are available to the public. This includes tracking adverse events, identifying potential risks, and taking action to protect public health. The FDA relies heavily on post-market surveillance to ensure that OTC drugs remain safe and effective over time.
17. If oral phenylephrine is ineffective, why is it still available for sale?
The FDA’s determination of ineffectiveness does not automatically result in a product being removed from the market. The FDA typically initiates a process that involves working with manufacturers to reformulate products or remove them from shelves. This process can take time, and consumers may still find products containing oral phenylephrine available for purchase in the interim.
18. How can I stay informed about updates regarding the FDA’s investigation into pseudoephedrine?
You can stay informed about updates regarding the FDA’s investigation by checking the FDA’s website for press releases and announcements. You can also follow reputable news sources that cover health and medical issues.
19. Are there any long-term studies on the effects of pseudoephedrine use?
There are not extensive long-term studies specifically focused on the long-term neurological effects of pseudoephedrine use at typical OTC dosages. The current concerns have arisen from post-market surveillance and case reports, prompting the FDA to conduct further investigation.
20. What are the implications of this news for the future of OTC decongestant medications?
The concerns surrounding pseudoephedrine and the ineffectiveness of oral phenylephrine may lead to increased scrutiny and regulation of OTC decongestant medications. This could result in more rigorous testing requirements, stricter labeling requirements, and potentially a reduction in the availability of certain products. It also highlights the need for ongoing research and development of safe and effective alternatives for treating nasal congestion.